WASHINGTON, Nov. 8, 2016 —
A clinical trial began here yesterday at the Walter Reed Army Institute of Research, where 75 participating healthy adults were vaccinated with a Zika virus vaccine that the institute’s scientists developed earlier this year, Walter Reed officials announced today.
The Phase 1 trial will test the safety and immunogenicity -- the ability of the vaccine to trigger an immune response in the body -- of the purified, inactivated Zika virus vaccine called ZPIV. The vaccine is being tested at WRAIR’s Clinical Trial Center in Silver Spring, Maryland.
“The Army has moved efficiently from recognizing Zika virus as a threat, producing ZPIV for use in animals and demonstrating its effectiveness in mice and monkeys, producing ZPIV for human testing, and now initiating clinical trials to establish its safety and build the case for subsequent efficacy trials,” Army Col. (Dr.) Nelson Michael, director of WRAIR’s Military HIV Research Program, or MHRP, and Zika program co-lead, said in a statement.
Efficacy refers to the vaccine’s ability to demonstrate a health effect when tested in a clinical trial.
“All of this,” he added, “was done in 10 months.”
Dr. Kayvon Modjarrad, Zika program co-lead and associate director for emerging infectious disease threats at WRAIR’s MHRP, said the Army was able to move so quickly in developing, manufacturing and testing a Zika vaccine “because of its extensive experience with this vaccine platform and longstanding investments in the understanding and mitigation of flaviviruses like yellow fever, dating back to the founding of WRAIR.”
DoD Zika Response
WRAIR officials say this study is part of the Defense Department response to the ongoing Zika outbreak in North and South America and Southeast Asia.
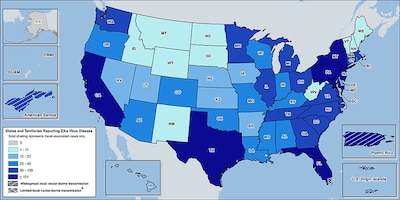
For service members, there are concerns about infection during deployment and travel, but also in the continental United States, where most military installations are concentrated in southern states. There, climate conditions and mosquito populations favor Zika transmission, WRAIR officials say.
As of Nov. 2, according to the Centers for Disease Control and Prevention, 149 cases of Zika infection were confirmed in the military health system, including four pregnant service members and one pregnant family member.
Zika infection during pregnancy, CDC says, can cause a birth defect of the brain called microcephaly and other severe fetal brain defects.
Other problems have been detected among fetuses and infants infected with Zika virus before birth, such as defects of the eye, hearing deficits and impaired growth. And reports have increased about Guillain-Barré syndrome, an uncommon sickness of the nervous system, in areas affected by Zika, CDC says.
But even Zika infections without symptoms “can lead to severe birth defects and neurological complications,” Zika study principal investigator Army Maj. (Dr.) Leyi Lin said, adding, “A safe and effective Zika vaccine that prevents infection in those at risk is a global public-health priority."
Zika and Other Flaviviruses
Flaviviruses like Zika are found mainly in mosquitoes and ticks and cause widespread morbidity and mortality worldwide. Other mosquito-transmitted viruses that are members of the flavivirus genus include yellow fever, or YF, dengue fever, Japanese encephalitis, or JE, and West Nile viruses, according to the CDC web page.
"We want to assess the safety and immune response of the ZPIV vaccine in JE and yellow fever YF vaccine recipients because these vaccines may alter the response to the ZPIV vaccine,” Lin said.
“Uniquely,” he added, “illness as a result of natural infection from JE, YF or Zika could be more severe when prior flavivirus infection or vaccination exists. Our study assesses co-vaccination to learn how to reduce risk when protecting against circulating flaviviruses.”
This is important for service members who are vaccinated against other flaviviruses and then stationed in or deployed to areas where Zika is becoming endemic, WRAIR scientists say.
Zika Vaccine Platform
WRAIR’s inactivated flavivirus vaccine platform was the same technology the institute used to create its Japanese encephalitis vaccine, licensed in 2009.
An earlier preclinical study found that rhesus monkeys vaccinated with ZPIV developed a strong immune response and were protected against two strains of Zika virus.
The National Institute of Allergy and Infectious Diseases, or NIAID, part of the National Institutes of Health, helped identify the viral strain used in the ZPIV vaccine, supported the preclinical safety testing and is sponsoring the conduct of this trial.
WRAIR, NIAID and the Department of Health and Human Services’ Biomedical Advanced Research and Development Authority, or BARDA, have established a joint research collaboration agreement to support the vaccine’s development.
The Pilot Bioproduction Facility at WRAIR manufactured the ZPIV vaccine being used in Phase 1 clinical studies, and the Army recently signed a cooperative research and development agreement to transfer the ZPIV technology to Sanofi Pasteur to explore larger-scale manufacturing and advanced development. BARDA recently awarded a six-year contract to Sanofi Pasteur to further develop this vaccine to licensure, according to the WRAIR release.
Other ZPIV Trials
WRAIR’s ZPIV candidate also will soon be part of an NIH trial that began in August. The NIH vaccine contains DNA that instructs volunteers’ cells to make certain Zika proteins that then illicit an immune response. As part of that study, WRAIR’s ZPIV vaccine will be given to volunteers as a booster after they receive the NIH DNA vaccine, WRAIR officials say.
Three more Phase 1 trials using ZPIV are scheduled to begin this year, the WRAIR release noted:
-- St. Louis University researchers, through the NIAID-funded Vaccine and Treatment Evaluation Units network, will examine the optimal dose of the vaccine to be used in larger studies.
-- Beth Israel Deaconess Medical Center and Harvard Medical School researchers will evaluate the safety and immune response from a compressed vaccine schedule.
-- The Ambulatory Center for Medical Research, part of Ponce Health Sciences University in Puerto Rico, will examine the vaccine’s safety and immune response in participants who have already been naturally exposed to Zika or dengue viruses.
The WRAIR trial that began yesterday is sponsored by NIAID and funded by the Army and the Defense Department.
(Follow Cheryl Pellerin on Twitter @PellerinDoDNews)