Dengue virus illustrated (Thomas Splettstoesser/Visuals Unlimited/Corbis) http://bit.ly/1Fz6kB3
Dengue, a mosquito-borne virus infects about 50 million people every year and kills several thousands. Recent reports from India suggests that outbreaks of Dengue fever have reached a 5-year high, resulting in several thousands infections and a few deaths. Currently there is no treatment for dengue and no vaccine available that offers complete protection.
However, there is Sanofi’s new dengue vaccine that has been approved for use and would hit the markets in the near future. The vaccine consists of an attenuated yellow fever virus with a dengue “coat”. However, the vaccine doesn’t offer the same level of protection against all four strains and varies from strain to strain. Thus, the search for a cure that will be equally effective against all four strains of Dengue namely DENV1, DENV2, DENV3 and DENV4, continues.
Two landmark papers published in the recent issue of Science Translational Medicine have uncovered some key insights which could pave way for better Dengue vaccines and therapeutics. Two teams of scientists, one at Australia’s University of Queensland and the other at University of California, Berkeley, led by Dr. Paul Young and Dr. Eva Harris respectively have found the secret of dengue’s virulence: a single protein, called nonstructural protein 1, or NS1. Dr.Young’s team found the mechanism by which NS1 operates, while Dr. Harris and team were able to isolate the protein itself and use it to vaccinate mice.
Dengue causes several symptoms such as fever, rash, muscle pain and damage to the blood vessels, which causes them to leak plasma. In severe cases, the fluid loss can be deadly, and the disease in its most serious form can become dengue hemorrhagic fever, which causes nausea, vomiting and bleeding or bruising under the skin. Once it infects people, they recover from it but have immunity only to that particular strain. Also, scientists were unclear about how the severe hemorrhagic form of the disease causes extensive damage and death.
Many hypothesized that severe cases of Dengue led the immune machinery of a person to go into an overdrive, leading to a cytokine storm. So, when a subsequent infection with another strain of dengue occurs, the antibodies from the first infection, binds to the new strain of dengue due to its similarity but ultimately fails to neutralize the virus completely. Instead, they allow the virus to attach to the T cells that would usually kill it, and that spreads the virus further, increasing the viral load on the patient.
This leads to more cytokine production, which makes the blood vessel walls more permeable, and when this goes into an overdrive, makes these blood vessels leaky. Tiny spots of blood appear on a patient’s skin and larger pockets of blood accumulate under the skin. This explains why infections the second time round are more severe.
Role of NS1 in Dengue fever
Dr. Young’s team had earlier done a study in Thailand which showed that patients who had high levels of NS1 were more likely to go on to severe disease states. So, they assumed it could probably be a viral infection marker, but they also wanted to investigate if it was directly influencing the course/severity of the disease.
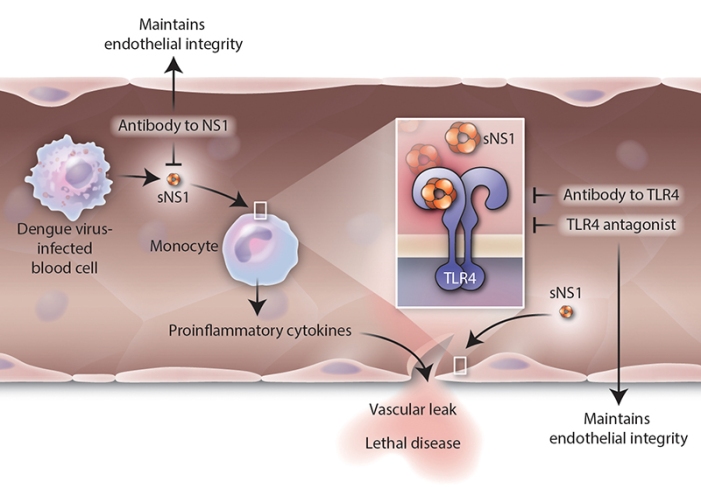
NS1 directly activates mouse macrophages and human immune cells via the innate-immune receptor TLR4. Secreted NS1 induced a dose-dependent increase in pro-inflammatory cytokines, disrupting endothelial cell monolayer integrity and leading to vascular leak. Blockade of TLR4, either by an antagonist or an antibody to NS1, maintained endothelial integrity and inhibited vascular leak. (H. McDonald/Science Translational Medicine) http://bit.ly/1Fz6kB3
Further research revealed that this protein NS1 binds to another molecule called toll-like receptor 4 (TLR4) that allows it to link to the cells lining the blood vessels, called endothelial cells. They also stimulated the body to release cytokines – the over-active inflammatory response suggesting that it acts very much like a bacterial toxin.
The second team at Berkeley, led by Dr. Eva Harris looked at NS1’s role in dengue infection more directly. They infected mice with both the dengue virus itself, and the protein itself (from all four strains of dengue). In both cases, the mice developed antibodies. They also discovered that, NS1 by itself (without the whole virus particle) can cause the blood vessels to leak fluid. “We thought that maybe the protein had a role in vascular leakage,” Harris says.
They fed the mice with a small amount of NS1 too, eliciting an immune response and they seemed to be protected from the virus. Dr. Harris suggests that the antibody links to the protein itself, rather than a specific viral strain. Moreover, NS1 produced by all four dengue strains is the same. Also, the protection against the virus wasn’t 100 percent across the different strains of dengue. In their study, Harris’ team found that when inoculated with the NS1 from DENV2, protection was 100 percent from that strain. It was 75 percent from DENV1, and 60 percent from DENV3 and DENV4.
Apart from mice, they tested the NS1 protein and the virus on human pulmonary endothelial cells in culture. They saw that NS1 wasn’t able to damage the cells when the TLR4 protein was blocked—more evidence that it is the NS1 that causes vascular leakage in humans.
Harris notes that their work, coupled with the findings of Young’s team that TLR4 links dengue to other cells, offers important insights. “If we can target TLR4, we have a new way of making a therapy,” she says, in addition to a vaccine. Their future plans include determining which specific piece of NS1 is the one that generates the right antibodies and damages cells.
Armed with this new information, public health officials can help better control the disease; as the usual methods are only focussed on controlling the mosquito.
One other advantage of using NS1 as a base for a vaccine is that it doesn’t involve using the virus at all. “The FDA would be happier if we could knock out pieces of the protein that cause disease and leave those that give protection,” Harris says.
The above article has been adapted from Smithsonianmag.com
The original articles can be accessed here: Dr. Paul Young group; Dr. Eva Harris group

No comments:
Post a Comment